AMINO ACID STEREO CONFIGURATION
Copyright: J. E. Wampler, 1996
Topics Covered:
Optical activity (quantified by the rotation of the plane of polarized
light as it passes through a substance) was measured long before
the three dimensional structure of molecules could be determined by
methods such as X-ray Crystallography. The experimental assignment of
optical activity was then symbolized with the letter "d" or the "+"
sign (for dextrotatory, right handed or clockwise rotation of the
plane of polarized light when viewed toward the light source)
and "l" or "-" (for levorotatory, left hand or counterclockwise rotation).
Later the "D" and "L" symbols were associated with absolute configuration
based on the arbitrary, but as it turned out, correct assignment of the
absolute configuration of the dextrotatory and levorotatory forms of
glyceraldehyde. In this symbolism, absolute configuration is based
on chemical synthesis starting with glyceraldehyde (Fischer and Rosanoff)
and optical activity is specified using the "+" and "-" notation.
 A second absolute configuration notation using the symbols R (from
rectus, latin for right) and S (from sinister, latin for
left) was developed by Cahn, Ingold & Prelog. In this approach, the
substituents on an assymetric carbon (eg. a tetrahedral carbon with
four different substituents) are prioritized by decreasing atomic number.
Configuration is assigned by "looking" down the bond to the lowest
priority substituent and assigning R to the configuration where the
remaining subtituents are arranged clockwise in decreasing priority.
S is then assigned to the molecular form where the substituents are
arranged counterclockwise.
A second absolute configuration notation using the symbols R (from
rectus, latin for right) and S (from sinister, latin for
left) was developed by Cahn, Ingold & Prelog. In this approach, the
substituents on an assymetric carbon (eg. a tetrahedral carbon with
four different substituents) are prioritized by decreasing atomic number.
Configuration is assigned by "looking" down the bond to the lowest
priority substituent and assigning R to the configuration where the
remaining subtituents are arranged clockwise in decreasing priority.
S is then assigned to the molecular form where the substituents are
arranged counterclockwise.
Some other rules for assigning R/S configuration:
Group Priorities:
SH > OH > NH2 > COOH > CHO > CH2OH > C6
H5 > CH3 > H
Larger groups are ranked at their points of divergence, i.e.
a CH2CH2SH is greater than a CH2CH2OH
The absolute stereo configuration of the amino acids at the alpha
carbon is typically
referred to using the D/L notation with reference to the absolute
configuration
of Glyceraldehyde rather than the more modern R/S designation. In R/S
notation change of a single substituent can change assignment. However,
all of the amino acids used in proteins (except for glycine which is not
optically active) are of L configuration. However, in R/S notation cysteine
is 2R, whereas the others are 2S.

Both threonine and isoleucine have a second assymetric carbon center
at carbon position 3 along their chains. The absolute configuration
of the normal protein component, L-threonine, is 2S,3R. The mirror image
compound (the enantiomer) is D-threonine (2R,3S). The diastereomer with
2S, 3S configuration is called L-allo-threonine (D-allo-threonine is then
2R, 3R). For isoleucine, the configuration of normal L-isoleucine is
2S, 3S.
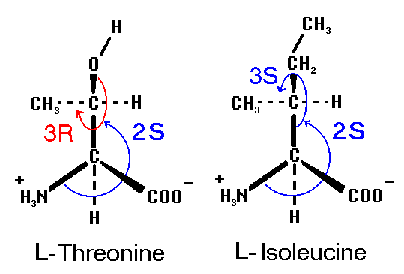
NOTE: While L-threonine and L-isoleucine have
different R/S configurations, the two molecules can be superimposed,
i. e. the arrangement of the methyl group, the hydrogen atom and the
backbone atoms are the same.
Return to tutorial.



