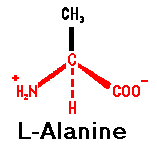
 Proteins are
polymers formed from alpha-amino carboxylic acids. The alpha-
carbon is typically optically active and, in proteins, in the
L-configuration.
Proteins are
polymers formed from alpha-amino carboxylic acids. The alpha-
carbon is typically optically active and, in proteins, in the
L-configuration.Copyright: J. E. Wampler, 1996
FIRST SOME DEFINITIONS:
 Proteins are
polymers formed from alpha-amino carboxylic acids. The alpha-
carbon is typically optically active and, in proteins, in the
L-configuration.
Proteins are
polymers formed from alpha-amino carboxylic acids. The alpha-
carbon is typically optically active and, in proteins, in the
L-configuration.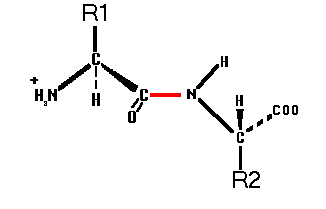 Two or more
amino acids covalently linked by an amide bond between the
carboxylic acid group of one and the alpha-amino group of the
other.
Two or more
amino acids covalently linked by an amide bond between the
carboxylic acid group of one and the alpha-amino group of the
other.
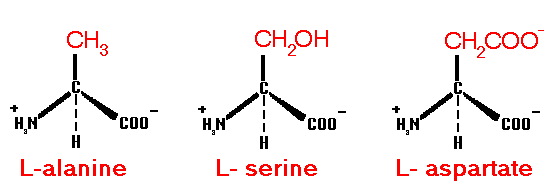
18 of the 20 commonly occurring, genetically encoded amino acids involved in protein structure have a primary alpha-amino group and an optically active alpha-carbon center. All also have an alpha hydrogen atom. The names of the amino acids then specify the fourth substituent attached at this position.
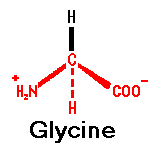

1 is optically active, but
has a secondary amine
IN ADDITION there are other amino acids in proteins:
Naturally occuring chemical modifications of the 20 genetically encoded amino acids, eg.
Hydroxylation of proline and lysinePhosphorylation of Serine & Tyrosine
R-group methylation of lysine, histidine and arginine.
R-group acetylation of lysine.
N-terminal methylation of alanine.
N-terminal acetylation of serine.
N-terminal formylation of methionine.
Addition of carbohydrates (at Asparagine and Serine).
Addition of Lipids (at Cysteine, N-terminal glycine and C-terminal via carbohydrate linker group)
Covalent attachment of cofactors (eg. pyridoxal-5-phosphate attached at lysine; hemes attached at cysteines).
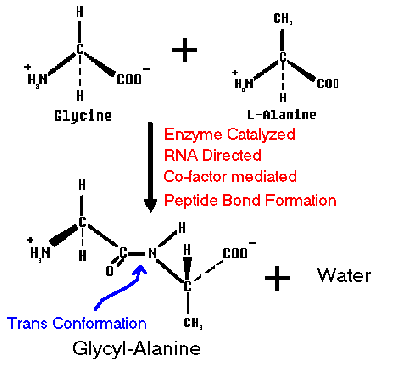
Thus the polymer formed is an unbranched chain of amino acids.
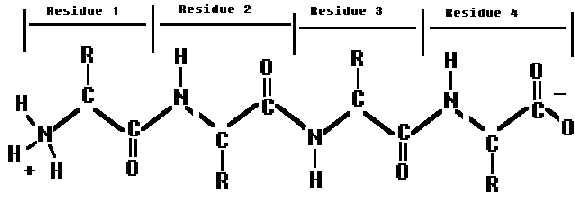 Always referred to from N-terminal(amino) to the C-terminal (carboxy).
Always referred to from N-terminal(amino) to the C-terminal (carboxy).
eg. Glycyl-alanyl-tyrosyl-glycineShort peptides are often given a general name according to how many residues (amino acids) are linked together.
Extended TRANS conformation (shown) where each torsion angle along chain is 180o
Glycyl-alanyl-tyrosyl-glycine is a "TETRAPEPTIDE"A general name for a long peptide is oligopeptide or POLYPEPTIDE.Alanyl-valyl-tryptophane is a "TRIPEPTIDE"
CYCLIC PEPTIDES are formed when an amide (or peptide) bond is formed between the N-terminal and C-terminal residues.
The most common covalent crosslink between peptide chains is the disulfide bond formed by two cysteine residues.
