
Copyright: J. E. Wampler, 1996
This section covers:
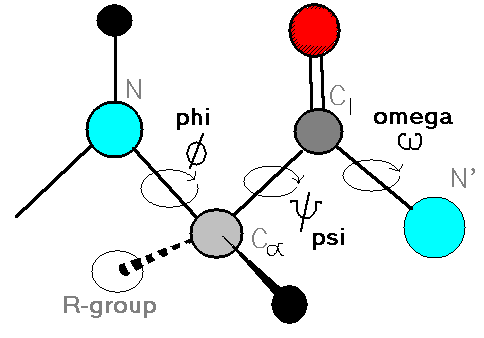
Definition of the torsion angles, phi, psi and omega.
Rotation about the amide bond.
Ramachandran plots and regular structure.
H-bonding patterns and regular structure.
Details of Alpha Helix.
Details of Beta Sheets.
Other structures with repetitive phi,psi angles.
Turns are identifiable structures too.
Since bond length and angles are fairly invariant in the known protein structures, the key to protein folding lies in the torsion angles of the backbone.
A torsion angles is defined by 4 atoms, A, B, C and D.

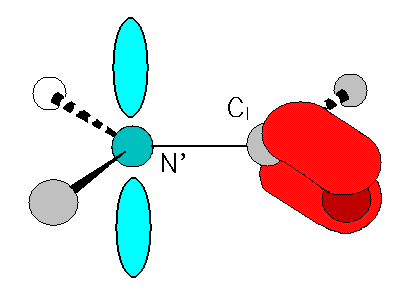
Trans is generally favored over cis:
Only 116 (0.36%) of 32,539 angles in 154 X-ray structures were found to be cis (Stewart et al. 1990).However...... some specific bonds are often cis, eg.
Tyr-Pro (25%), Ser-Pro (11%), X-Pro (6.5%)This leaves phi and psi for flexible folding of the chain. However, steric conflicts limit even these angles as well.
Repeating values of phi and psi along the chain result in
regular structure. For example, repeating values of phi ~-57o
and psi ~-47o give a right-handed helical
fold (the alpha-helix). The structure of cytochrome C-256
shows many segments of helix and the Ramachandran plot shows
a tight grouping of phi,psi angles near to -50,-50.


The D-H interaction is characterized by:
Polarization due to electron withdrawal from the hydrogen to D giving D partial negative charge and the H a partial positive charge.The proximity of the Acceptor A causes further charge separation.These separated partial charges make the D-H bond a dipole.
Shortened D-H bond distance (typically ~ 1 Angstrom).
A decreased Hydrogen radius (from 1.2 to ~ 1 Angstrom).
The result is:
Closer approach of A to the hydrogen (typically ~ 2 Angstroms).In proteins and nucleic acids the predominant regular structures are also characterized by repeating hydrogen bond opportunities. With protein secondary structure, regularly spaced intrachain hydrogen bonding is seen.Higher interaction energy than a simple van der Waals interaction.
A point-charge to dipole type interaction which...
"reaches" further (~1/(distance)3 or 1/(distance)2) than a typical van der Waals interaction (~1/(distance)6)AND
has angle dependence (a point-charge to dipole interaction falls off as the cosine of the angle that the A to H vector makes to the D-H vector).
 The alpha helix is characterized by
hydrogen bonds along the chain, almost co-axial. When the phi,psi
angles are in the nominal range:
The alpha helix is characterized by
hydrogen bonds along the chain, almost co-axial. When the phi,psi
angles are in the nominal range:
- the mainchain carbonyl C=O of each residue H-bonds to the amide N-H 4 residues along the chain- there are about 3.6 residues per turn of the helix
- the sidechain are projected outward
 The beta sheet is characterized by
hydrogen bonds crossing between chains. Beta sheet strands are:
The beta sheet is characterized by
hydrogen bonds crossing between chains. Beta sheet strands are:
- not fully extended (phi,psi = -180,180) due to sidechain steric inteferences- the chain is slightly "puckered" so that the sheet is sometimes said to be "pleated"
- two H-bonding chains may run parallel or antiparallel
- on average parallel (both chains running N-terminal to C-terminal in the same direction) sheet chains have phi,psi = -119,113.
- on average antiparallel sheet chains have phi,psi - -139,135.
Name phi psi Comments
------------------- ------- ------- ---------------------------------
alpha-L 57 47 left-handed alpha helix.
3-10 Helix -49 -26 right-handed.
pi helix -57 -80 right-handed.
Type II helices -79 150 left-handed helicies
formed by polyglycine
and polyproline.
Collagen -51 153 right-handed coil formed
of three left handed
helicies.
Turns involving four (4) residues are more common with hydrogen bonding from the carbonyl of residue 1 to the N-H of residue 4. One class of these turns is called beta-turns, typically found at turns of beta-sheet structure and designated by Roman numerals. Some of their characteristics are:
Residue 2 Residue 3
Designation Phi,Psi Phi,Psi Comments
------------- ---------- ---------
I -60,-30 -90,0 Most common type.
II -60,120 80,0
III -60,-30 -60,-30 Like 3,10 helix.
IV*
V -80,80 80,-80
VIa -60,120 -90,0 2-3 peptide bond
is cis, residue
3 is proline.
VIb -120,120 -60,0 2-3 peptide bond
is cis, residue
3 is proline.
VII**
VIII -60,-30 -120,120
---------------------------------------------------------------
* Type IV is for turns not otherwise classified
** Type VII is a bend recognized by psi(2)~180 and phi(3)<60 or
by psi(2)<60 and phi(3)~180.
Favorable and (unfavorable) Residues in Beta-turns by Position:
Type Residue 1 Residue 2 Residue 3 Residue 4
-------- ----------- ----------- ----------- -----------
I Asp, Asn, Pro (Pro) Gly
Ser, Cys
II Asp, Asn, Pro Gly, Asn Gly
Ser, Cys
VIa Pro
VIb Pro
---------------------------------------------------------