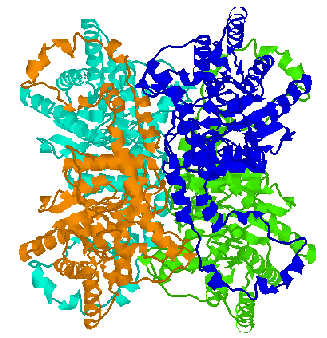
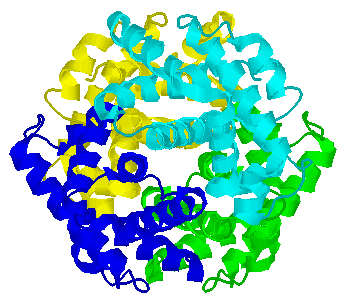
Some proteins are composed of identical subunits (chains). A simple example is the dimer of HIV Protease.
Copyright: J. E. Wampler, 1996

Some proteins are composed of identical subunits (chains). A simple example
is the dimer of HIV Protease.
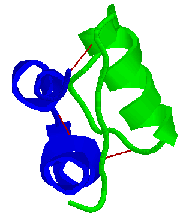 Some proteins are composed of non-identical subunits (chains). A simple
example is insulin which is made up of two chains, the alpha chain and
the beta chain, linked by two disulfide bridges.
Some proteins are composed of non-identical subunits (chains). A simple
example is insulin which is made up of two chains, the alpha chain and
the beta chain, linked by two disulfide bridges.
Even with identical subunits, their relative positioning controls the
symmetry of the complex and can have biochemical implications. Consider
two right hands, nearly identical to each other but completely assymetric.

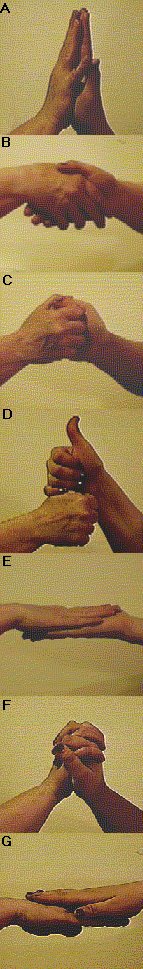
They can be put together to form a "dimer" in many different ways. In some complexes, both "subunits" touch the other one in exactly the same way (Panels A,B,C,E and F) forming an interface with matching surfaces. The result is referred to as an "ISOLOGOUS" association.In others (Panels (D and G) they each experience a different contact referred to as a "HETEROLOGOUS" association. In these cases, even though they are identical in shape, they are not identical in environment.
These hands also illustrate some other variations seen in protein complexes:
Some complexes involve compact subunits that interact along well defined surfaces (eg. Panels A, E & G).With more subunits in the complex, we can imagine some emergent properties that can have biochemical implications. For example, with three identical subunits arranged linearly, the middle subunit experiences a very different environment from the other two.Some complexes involve more intimate and complex interactions and in a few cases protein chains are interwoven (eg. Panel F).
Some arrangements are ideal for further polymerization. We can imagine as stack of "subunits" of any number built on the basis of the heterologous interactions in panels D and G, or a stack of an even number of subunits based on isologous interactions like say those of Panel C.
The possibilities grow with the number of subunits.